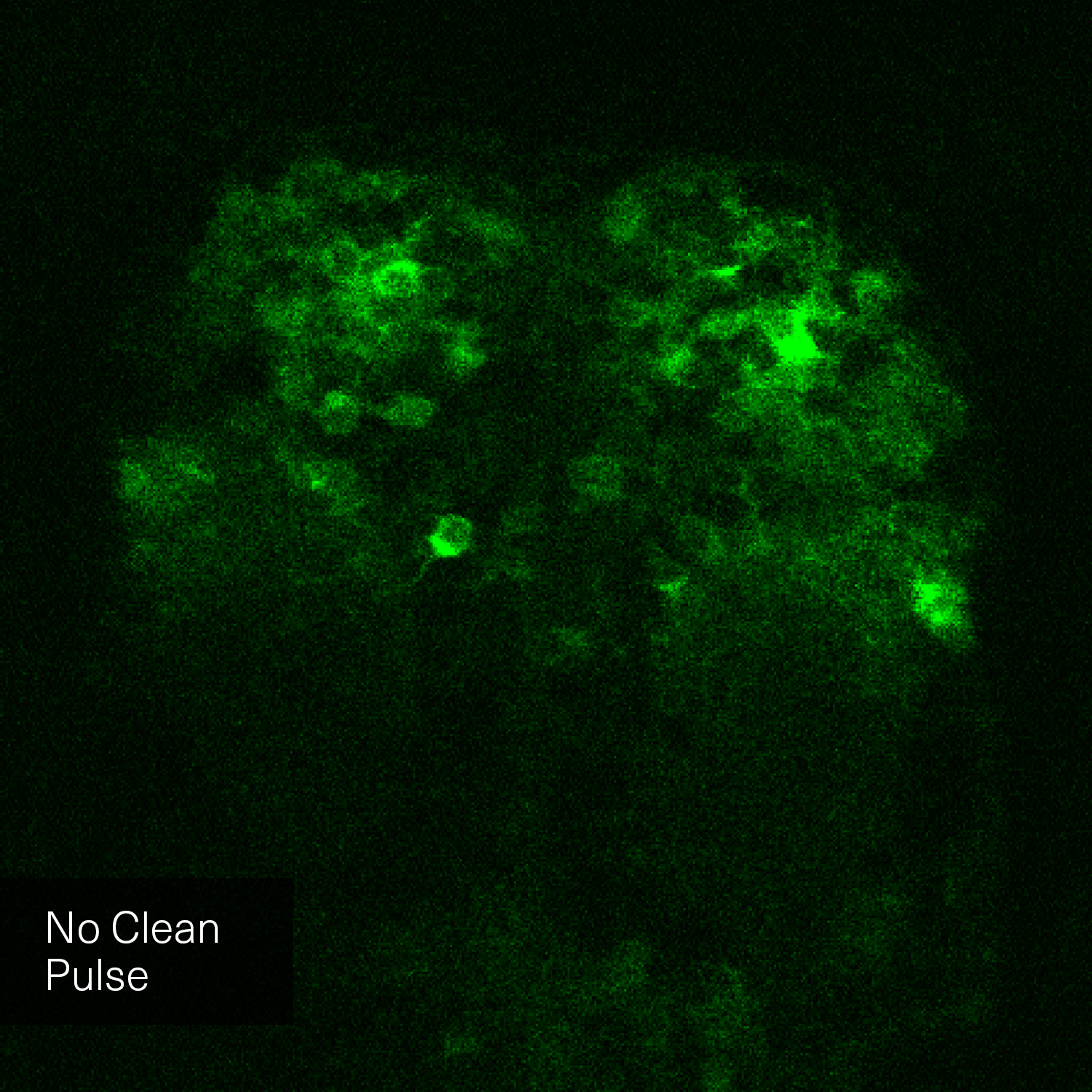
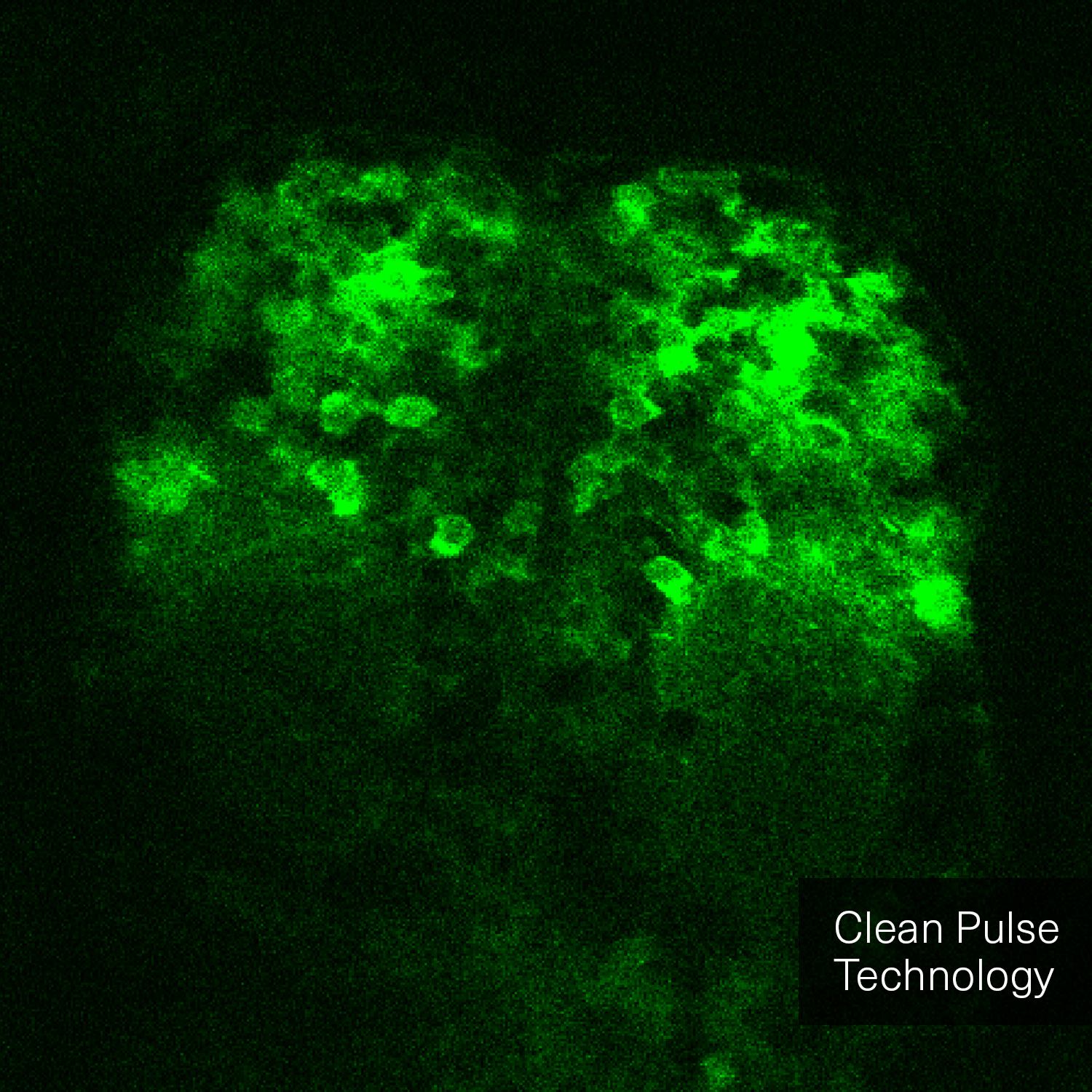
Clean Pulse Technology
Brighten up your images with TOPTICA’s femtosecond fiber lasers
Multi-photon microscopy is a nonlinear microscopy technique where multiple near-infrared photons interact with the sample to create a fluorescent excited state. Thus, the fluorescence brightness and contrast when imaging depend on the peak-power of the excitation laser. The peak-power is determined by the average laser power, the repetition rate, the pulse duration at the sample plane and most importantly the temporal pulse quality.
During in-vivo imaging, the laser average power should be limited at the sample plane to prevent thermal damage and phototoxicity. With the typical throughput of a multi-photon microscope, a Watt-level laser has more than sufficient power for 2-photon excitation. In addition, 80 MHz is a well-established repetition rate yielding appropriate pixel dwell times and synchronization possibilities with galvo and resonant scanners.
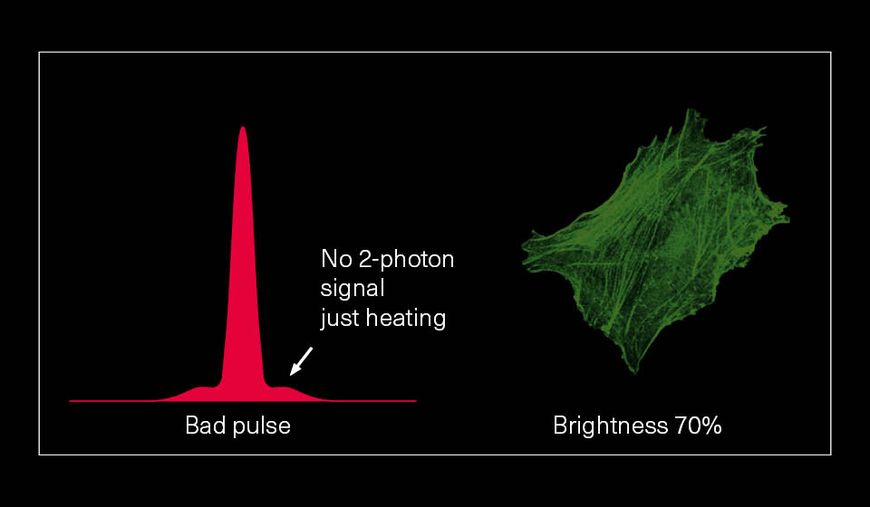
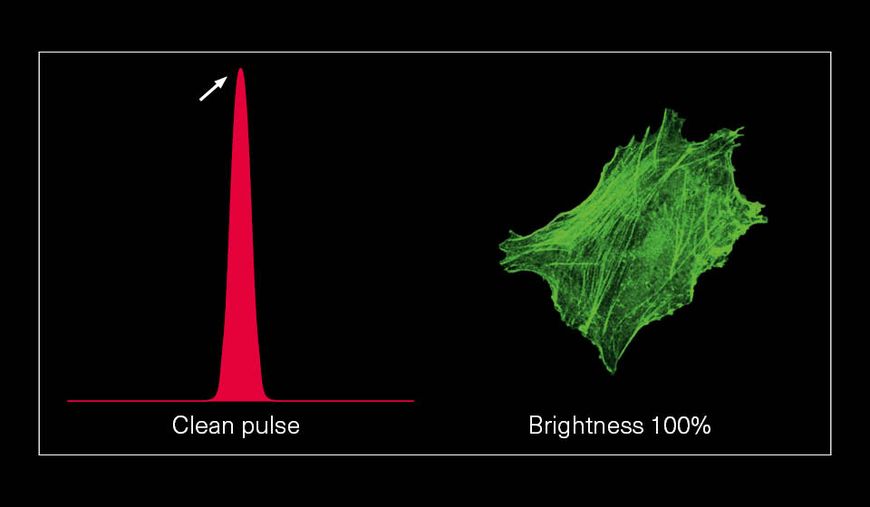
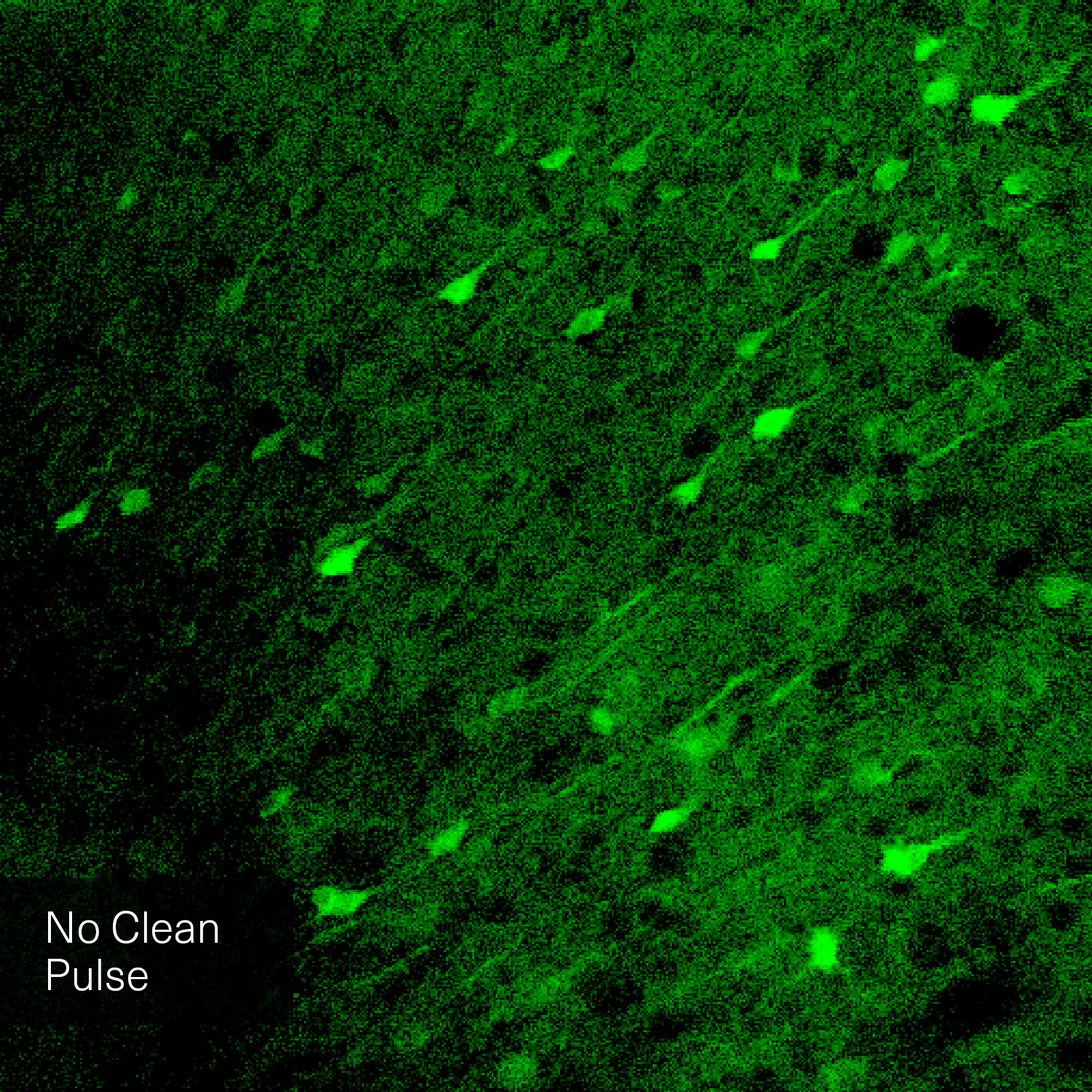
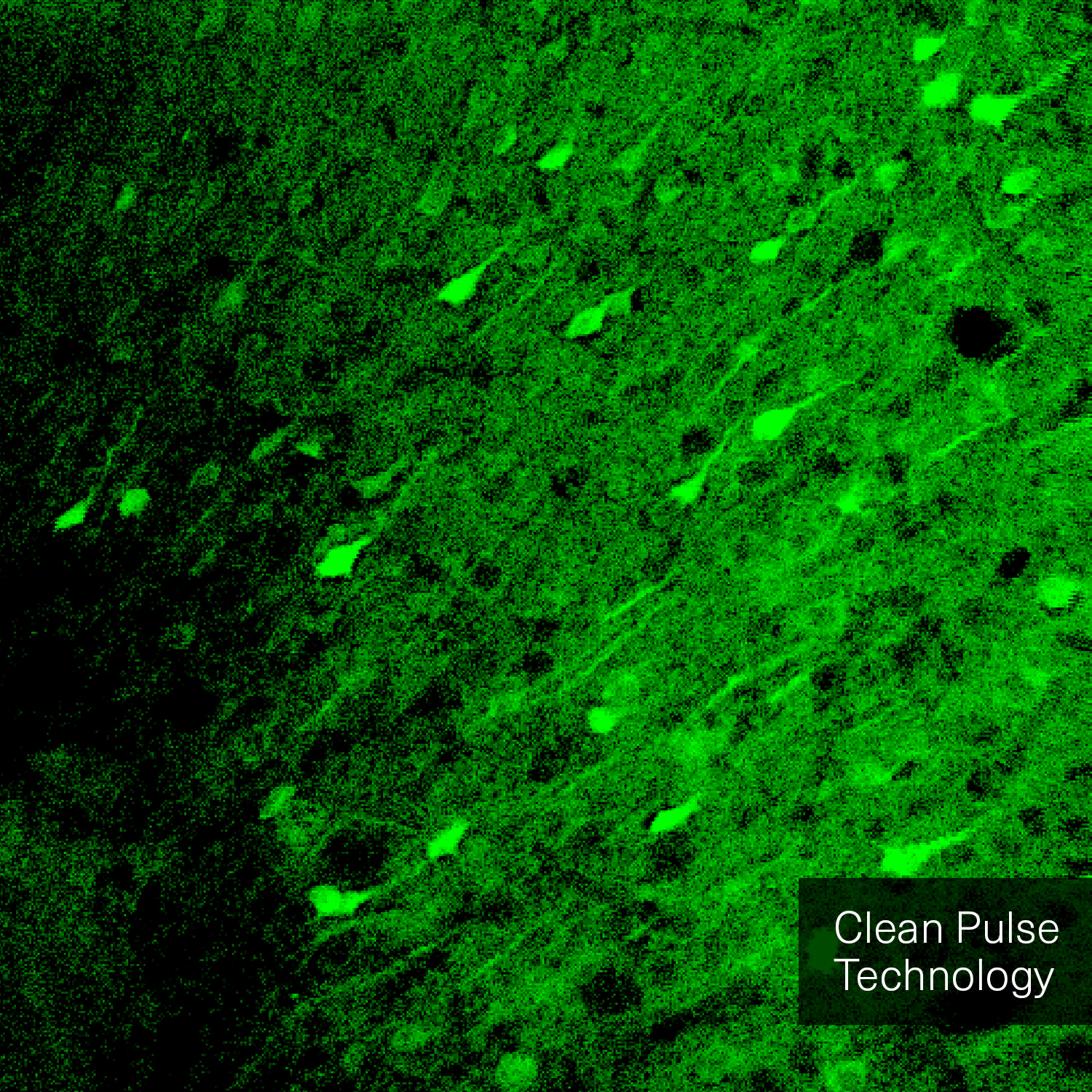
Hence, with laser power and repetition rate fixed by experimental requirements, pulse duration and temporal pulse quality are the crucial factors that can be optimized for fluorescence image quality. Pedestals and side-wings on the temporal pulse profile do not contribute to the non-linear absorption but only unnecessarily heat the sample. Likewise, shorter pulses yield higher fluorescence intensity due to higher peak power. Thus, the combination of a high temporal pulse quality (a “clean pulse”) and a short pulse duration at the sample plane gives the best result in terms of overall 2-photon excitation. This ultimately improves the contrast and brightness of fluorescence images.
The pictures from the article Influence of laser pulse shape and cleanliness on two-photon microscopy by Shau Poh Chong and Peter Török are used with permission of the authors.
TOPTICA's offer
To achieve the best image quality, TOPTICA’s FemtoFiber ultra 920 incorporates Clean Pulse Technology - a laser design that significantly reduces pedestals and side-wings on the temporal pulse profile compared to other laser amplifier designs. Thus, the full laser power of the FemtoFiber ultra 920 is contained in the main laser pulse to maximize two-photon excitation. With Clean Pulse Technology, the FemtoFiber ultra 920 enables unmatched fluorescence image brightness in non-linear microscopy (see Literature).
Additionally, TOPTICA’s FemtoFiber ultra provides motorized group delay dispersion (GDD) pre-compensation that can be controlled in software. Due to chromatic dispersion, a femtosecond pulse stretches in time when transmitted through optical material in the microscope. GDD pre-compensation adds the opposite dispersion to circumvent this effect by cancelling it out. TOPTICA’s software-controllable GDD pre-compensation ensures short pulses at the sample plane with user-friendly, repeatable optimization of fluorescence signal strength via the graphical user-interface (GUI).
-
Related Products
-
Literature
- Scientific Article: Shau Poh Chong and Peter Török, "Influence of laser pulse shape and cleanliness on two-photon microscopy," Opt. Continuum 3, 552-564 (2024)
- Scientific Article: Samir Saidi, Matthew Shtrahman, “Evaluation of compact pulsed lasers for two-photon microscopy using a simple method for measuring two-photon excitation efficiency“, Neurophotonics, Vol. 10, Issue 4, 044303 (November 2023)
- Scientific Paper: Simplifying two-photon microscopy (2020)
- Article: TOPTICA Introduces FemtoFiber ultra 920 (2019)